من جامعه اوهايول
حيث تقبل الافكار والمقتراحات من الطلبة والوصول الى النتائج الممتازة
نجح فريق العمل فى جامعه اوهايو الامريكية Ohio University
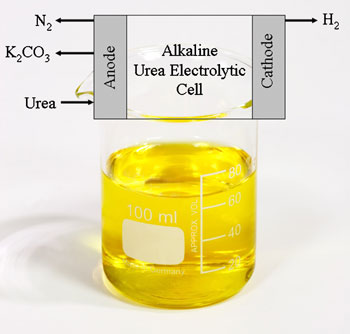
فى التوصل الى معدن جديد من النيكل an inexpensive new nickel-based electrode to selectively and efficiently oxidise the urea
يستطيع تحليل البول والحصول على غاز الهيدروجين منه باقل كهرباء
ويستخدم مصدر للكهرباء لايزيد عن 1.23V needed to split water
لتحصل على الهيدروجين
وهذه طفرة فى طرق الحصل على الهيدروجين
ويعمل هذا الفريق على الوصول الى خلية تحليل للبول يمكنها انتاج كميات كبيرة جدا من الغاز تكفى لتشغيل كل انواع السيارات
للمزيد
[B]Producing hydrogen from urine[/b]
July 3rd, 2009 [IMG]http://www.physorg.com/newman/gfx/news/1-urine.jpg[/IMG][Enlarge](http://www.physorg.com/newman/gfx/news/hires/1-urine.jpg)
Sample of urine. Image: Wikipedia
[B](PhysOrg.com) -- You do two things at motorway services: fill up one tank and empty another. US chemists have combined refuelling your car and relieving yourself by creating a new catalyst that can extract hydrogen from urine.[/b]
[Ads by Google](http://services.google.com/feedback/abg?url=http://www.physorg.com/news165836803.html&hl=en&client=ca-pub-0536483524803400&adU=www.ocas.be/&adT=Hydrogen+%26amp%3B+steel+choice&adU=FlowReact.com&adT=Modern+Hydrogenation&done=1)
Hydrogen & steel choice - Test influence of hydrogen on steel Testing facility + steel selection - www.ocas.be/
Modern Hydrogenation - Flow Hydrogenation is Faster, Easier and 100% Safe. Free D/B! - FlowReact.com
[I]Chemistry World[/i] reports that the catalyst could not only fuel the hydrogen-powered cars of the future, but could also help clean up municipal wastewater.
Gerardine Botte of Ohio University uses an electrolytic approach to produce hydrogen from urine - the most abundant waste on Earth - at a fraction of the cost of producing hydrogen from water.
Urine’s major constituent is urea, which incorporates four hydrogen atoms per molecule - importantly, less tightly bonded than the hydrogen atoms in water molecules.
Botte uses electrolysis to break the molecule apart, developing an inexpensive new nickel-based electrode to selectively and efficiently oxidise the urea. To break the molecule down, a voltage of 0.37V needs to be applied across the cell - much less than the 1.23V needed to split water.
Her work is described in the Royal Society of Chemistry journal Chemical Communications.
“During the electrochemical process the urea gets adsorbed on to the nickel electrode surface, which passes the electrons needed to break up the molecule,” Botte told Chemistry World.
Botte believes the technology could be easily scaled-up to generate hydrogen while cleaning up the effluent from sewage plants. “We do not need to reinvent the wheel as there are already electrolysers being used in different applications.”
More information: B K Boggs, R L King and G G Botte, Chem. Commun., 2009, DOI: 10.1039/b905974a
Provided by Royal Society of Chemistry
الرابط للخبر
الصور بالمرفقات[/b]